FEBRUARY 2024

Past Editions—Need to Know Archives
In this edition:
-
New York law to age-restrict some dietary supplements takes effect in April; CRN working to mitigate impact
-
Government report on prenatal vitamins raises unnecessary alarm—’worst outcome’ is if it dissuades young women from using them
-
FDA warns against dangerous tianeptine-containing products; they are not dietary supplements, CRN says
-
New Economic Impact Study quantifies positive impact of supplement industry across the U.S.
-
Free ‘Dietary Supplements 101’ webinar Thursday
New York law to age-restrict certain dietary supplements takes effect in April; CRN working to mitigate impact

New York Gov. Kathy Hochul signed into law A.5610/S.5823 in October 2023 despite strong opposition from CRN and instate retailers, as previously reported in an alert to our “Need to Know” retailer newsletter recipients.
Slated to go into effect on April 22, the new law will prohibit the sale of weight loss and sports nutrition dietary supplements to New York consumers under the age of eighteen.
-
The law restricts sales of supplements that are “labeled, marketed, or otherwise represented for the purpose of achieving weight loss or muscle building” and requires that retailers obtain age verification at the point of sale.
-
Online and direct selling retailers are required to ship the product using a service that verifies age upon delivery. The legislative record also indicates that one of the bill sponsors believes that the Act requires online retailers to verify ID online at the point of sale.
-
Retailers may need to consider how they shelve and store the affected dietary supplements because failing to comply with these new restrictions could result in costly fines.
A special interest group called the Strategic Training Initiative for the Prevention of Eating Disorders (STRIPED) is the driving force behind age-restriction proposals across several states. The group’s rationale relies on faulty science that scapegoats the dietary supplement industry by claiming an unfounded, and scientifically debunked, causal relationship between dietary supplement use and eating disorders and/or body dysmorphia in young adults.
CRN met with state officials in January seeking to mitigate the law’s impact on safe and legal dietary supplements. Subsequently, CRN’s board of directors is evaluating other options to protect retailers from implementation of the law.
“This will impact a huge swath of products sitting on grocery store, pharmacy and natural retailer shelves throughout New York State,” said CRN President & CEO Steve Mister. “Because of this blatant, alarmist misinformation pushed by STRIPED, Empire State consumers of all ages will have their ability to purchase dietary supplement products limited. It’s a blow to consumer choice and scientific accuracy, and our membership needs to understand just what a severe threat STRIPED poses to our industry.”
Supplement retailers are encouraged to contact CRN for more information about how to comply with the New York law, how to determine which products are impacted, and how to join efforts to block the legislation in other states.
Government report on prenatal vitamins raises unnecessary alarm

Retailers selling prenatal supplements need to be aware of a recent Government Accountability Office’s (GAO) report, “Prenatal Supplements: Amounts of Some Key Nutrients Differed from Product Labels.”
The GAO report asserts that 11 out of 12 prenatal supplements that it tested in 2023 by a contract laboratory deviated from their labeled amounts for at least one nutrient (folic acid, iodine, iron, vitamin A, C, or E).
-
The report does not disclose how the products were tested or which third-party testing lab performed the study, which is critical to better understand the study’s findings.
-
GAO also tested the 12 prenatal supplements for the presence of heavy metals and found that none of them contained amounts likely to cause a health concern based on accepted metrics used by FDA.
What they’re saying: The report concluded, “The limited reach of FDA’s oversight over dietary supplements—including prenatal supplements—could lead to unfavorable health outcomes for vulnerable populations (i.e., pregnant individuals and fetuses).” Further, the GAO recommended that “Congress consider measures for allowing FDA sufficient authority to carry out its oversight of dietary supplements. FDA agreed with this matter.”
CRN agrees with the report’s final recommendation that Congress should make FDA “require dietary supplement manufacturers to notify or register with FDA prior to putting any dietary supplement on the market and to provide a copy of the supplement label.” In fact, CRN members provide that information in the Supplement OWL as a condition of membership.
Retailers can share helpful tips for consumers looking to purchase supplements, such as looking for third-party seals or certifications on products and more.
CRN noted in a statement that the GAO report raises unnecessary alarm. CRN cautioned that the report should not dissuade young women from talking with their doctors about the importance of prenatal vitamins for a healthy pregnancy. Read more in coverage from WebMD, Healthnews, and NutraIngredients-USA.
FDA warns against dangerous tianeptine-containing products, widely known as ‘gas station heroin’; they are not dietary supplements, CRN says

Photo credit: FDA
FDA issued warning letters in January alerting the public to potentially dangerous tianeptine-containing products “marketed as dietary supplements,” and warning retailers not to sell it in order “to keep consumers safe.” The agency also announced that the manufacturer would voluntarily recall the products. A Congressional inquiry into tianeptine’s presence in the U.S. is ongoing, as reported by USA Today.
-
Tianeptine, also known as tianeptine sulfate, tianeptine sodium powder, Tianaa, Tianna Green, Tianna Red, and Tianna White, is used as a prescription antidepressant in some countries, but is not approved in the U.S. for any medical or commercial use. It is not a legal dietary ingredient.
CRN President & CEO Steve Mister, in a recent Natural Products Insider byline, called on the industry and other stakeholders to combat illegitimate products in the marketplace by carefully curating their supplement offerings to avoid illegal ingredients; putting pressure on FDA for stricter enforcement; and supporting legislative solutions to remove illegal products from the market.
The bottom line: Retailers are urged to prioritize the health and safety of customers by ensuring all products sold are compliant with FDA regulations. This could involve more rigorous vetting of suppliers, staying informed about FDA warnings and recalls, and taking swift action to remove problematic products from shelves.
Go deeper: View CRN’s statement warning consumers against purchasing tianeptine-containing products.
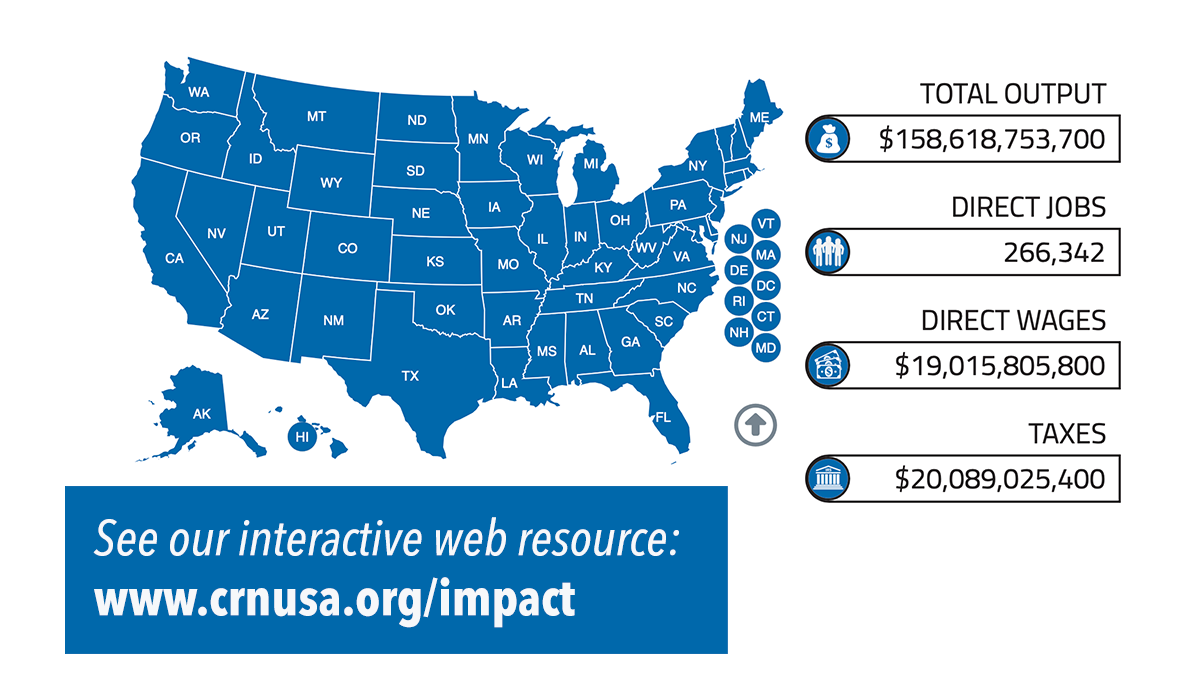
New Economic Impact Study quantifies positive impact of supplement industry on U.S., local communities
CRN’s recently released Economic Impact Study reveals the dietary supplement industry’s positive impact on jobs, wages, taxes, and other financial contributions in all 50 states, and in Congressional districts. The industry drives nearly $160 billion in economic output to support the nation’s economy.
Why it matters: The study data allows CRN to specifically quantify and communicate to legislators and constituents the financial importance of supplement companies within each legislative district and help Congress understand the impact of legislation on these businesses.
By the numbers: The dietary supplement industry, an essential component of the U.S. economy, provides jobs to more than 615,000 individuals and contributes significantly to public finances. In 2023, the industry's economic contribution reached $159 billion, marking a 23% rise from $121.6 billion in 2016.
“In the eight years since our last report, the industry’s footprint and subsequent impact to the local, state and national economies has increased dramatically and we believe it is important for the public and elected officials to know the positive contributions our member companies have on communities all over the country,” said CRN President & CEO Steve Mister.
“In some suburban or rural areas, they are among the largest employers and taxpayers, if not the largest, and provide needed jobs and revenue. This report helps us clearly quantify that impact in specific and tangible terms.”
The big picture: CRN's Economic Impact Study provides critical insights that can help dietary supplement retailers in strategic planning, advocacy, market analysis, and more.
Go deeper: Learn more about the study in a recent press release and check out the industry’s impact in your district.
Free ‘Dietary Supplements 101’ webinar Thursday
Key factors driving retail success in the dietary supplement space will be explored this Thursday during a complimentary webinar from Natural Foods Merchandiser.
Hear from CRN’s Michael Meirovitz, senior director, government relations, along with other experts who will discuss:
-
Trending supplements categories
-
Best practices for staff and consumer education
-
Regulatory challenges and more
Register here and log on Thursday, Feb. 15, at 12 pm ET / 9 am PT.
Questions? Contact Elise Hall.