OCTOBER 2023

Past Editions—Need to Know Archives
In this edition:
-
CRN survey shows essential role of supplements for consumers
-
FDA Commissioner Califf doesn’t use supplements, questions claims, industry efforts
-
December summit to convene retail, manufacturing stakeholders
-
Update on NY age restriction legislation
CRN Consumer Survey finds most users agree supplements are essential to maintaining health
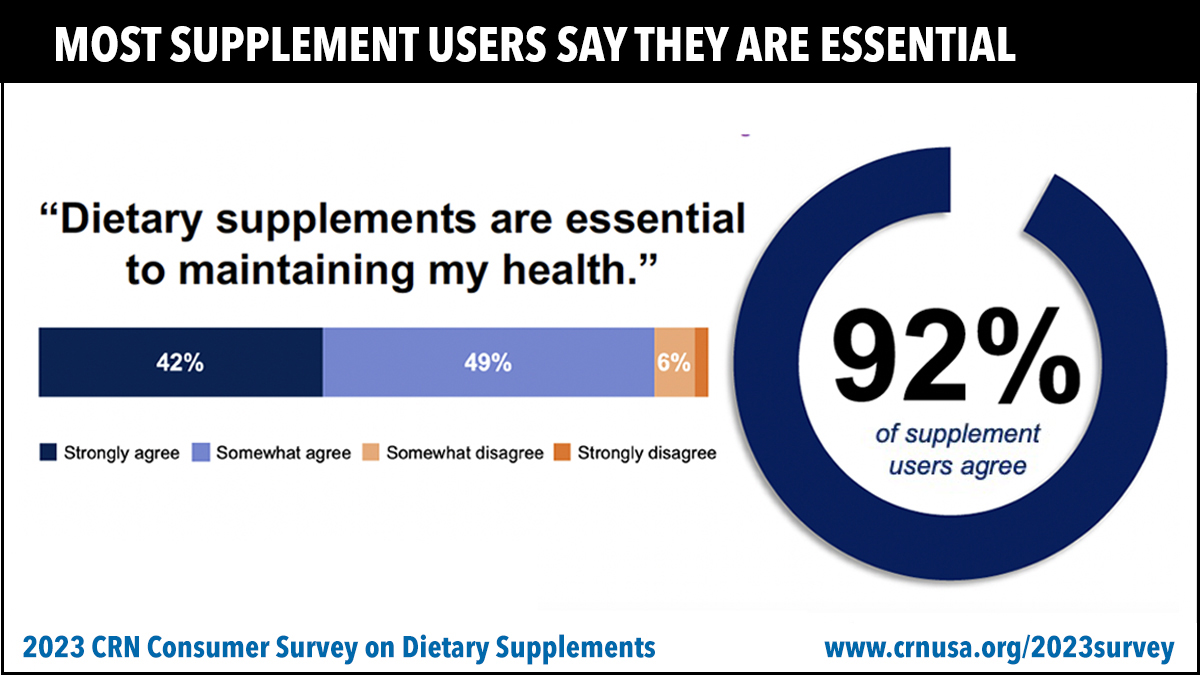
Topline data from the 2023 CRN Consumer Survey on Dietary Supplements confirms the mainstream status of supplements and the important role they play in the lives of most Americans.
By the numbers:
-
74% of U.S. adults report taking dietary supplements—about the same as 2022
-
55% consider themselves “regular” users
Further analysis of the data reveals that people are using supplements not only to fill nutrition gaps they acknowledge persist, but to support their more active and healthy lifestyles and their aspiration for optimal wellness.
-
92% of users agree that dietary supplements are essential to maintaining their health, according to responses to a new question included this year in the survey.
“Dietary supplements have become a non-negotiable component of so many Americans’ health habits—and this year’s survey data illustrates a consumer who recognizes that dietary supplements are vital to living the life they want to live,” said CRN President & CEO Steve Mister. “These findings may provide some reassurance about the durability of supplement usage despite economic unpredictability.”
Conducted annually since 2000, the survey serves as the leading resource for data on consumer attitudes about, and usage of, dietary supplements. Mass market retailers dominate with 93% of supplement purchasers saying they bought supplements from this channel within the past year. Twenty-five percent of users said they purchased online, with 77% of those online shoppers buying from Amazon; only 22% of those online shoppers say they purchased from a supplement company’s website.
Go deeper: See CRN’s press release for more topline findings. The full dataset is available to retailers for purchase.
FDA Commissioner Califf doesn’t use *any* supplements—questions claims, and industry self-policing during conference fireside chat

FDA Commissioner Robert M. Califf, M.D., joined CRN President & CEO Steve Mister for a virtual fireside chat during the opening session of the association’s Now New Next conference earlier this month.
What they’re saying: “When I walk down the aisle, I see a lot of these structure/function claims, and I have no idea what they mean,” said Dr. Califf. He volunteered that he does not use supplements, not even a multivitamin.
-
“Maybe you understand it—you’ll have to explain it to me one day. In my view, if you ingest something, it either makes your health better, it makes it worse, or it has no effect. And we know how to measure whether health is getting better. It's not a secret. The structure/function claim, that's the law and so we're going to play by the rule book.”
Mister pressed the Commissioner, speaking of claims and playing by the rule book, on the matter of enforcement against bad actors.
-
“The Food Drug & Cosmetic Act says that the claims that are made on dietary supplement labels have to be truthful and not misleading to consumers. They have to be substantiated,” Mister said. “What is the role of FDA where you have these products in the market that are clearly illegal and the industry doesn't have the guns and badges to enforce it? We can do things for ourselves, but we can't enforce it against the bad actors.”
Dr. Califf responded with a call for more industry support for getting FDA resources. “We’ve got industries that come to the table and work hard to get the support that's needed for FDA to allocate the people that are needed to get the job done. And I'm not seeing that from your industry side.”
Mister pushed back with facts about the dietary supplement industry’s robust and effective efforts in support of FDA.
-
“It was the industry that very much pushed in the 2015–16 timeframe to elevate the Office of Dietary Supplement Programs,” Mister said, recalling it was previously a division of the Office of Nutritional Products, Labeling, and Dietary Supplements.
-
“We pushed very hard to get it elevated to an office level,” Mister added. “And then for the last seven years, every year the industry has gone to bat to get more appropriations. And we have more than doubled the amount of designated appropriations from Congress to dietary supplement issues.”
Mister reiterated concerns about FDA’s proposed reorganization that could undermine years of building an infrastructure at the agency to increase the regulation and enforcement.
Dr. Califf replied that “this is not going to be made smaller in terms of the efforts at FDA... you’ve got a $60 billion industry. It's got a lot of good people in it, but it's got some bad players. We've got to work together so that the good players get rewarded, the bad players get punished.”
Go deeper: Hear analysis of Commissioner Califf’s comments from CRN SVP and General Counsel Megan Olsen in an interview with NutraIngredients-USA.com as well as the publication’s “Top 6 Takeaways” and coverage from Natural Products Insider.
Join the conversation on harmonizing retailer standards at GRMA Summit in Boca Raton this December

The Global Retailer and Manufacturer Alliance (GRMA) will hold its 2023 summit in Boca Raton, Florida, Dec. 5–8.
-
The GRMA annual summit provides retailers the opportunity to discuss quality and regulatory insights, collaborate and contribute to industry practices, and will serve as a platform to strengthen the quality within the supply chain for the health and wellness industry.
Why it matters: Retailers can be the last line of defense when it comes to protecting consumers, however, individual retail quality standards programs should not be weaponized against each other, creating unnecessary burdens on product manufacturers. Dietary supplement products on the shelves should be safe no matter what retail establishment they are purchased in—and harmonization of standards is paramount.
Learn more and register here.
NY age restrictions await Governor’s signature

As previously reported, age restriction legislation in the state of New York has passed both the Assembly and the Senate and sits with Governor Kathy Hochul for final signing into law.
CRN and its members continue to express opposition to the legislation with the Governor’s office, including that it unfairly targets traditional brick-and-mortar stores because the age restrictions are unenforceable against online retailers.
CRN will keep stakeholders updated on the status of the legislation and remains poised to push back against similar proposals that could surface in other states. Developing solutions for state-level age restrictions is one of CRN’s priority initiatives in 2023.

