Q1 2025 REPORT | Q2 2025 REPORT | Q3 2025 REPORT
CRN closed out a prolific year of advancing the dietary supplement and functional food industry by delivering value to its members in Q4 across multiple disciplines:
- responding to high-profile media coverage with evidence-based context;
- convening experts for intel and action on emerging business, science, and regulatory issues;
- reinforcing science-based safety standards;
- opposing state age restrictions; and more.
Download printable one-pager here.

CRN MAINTAINS CALL FOR CONSTITUTIONAL PROTECTIONS IN NEW YORK AGE-RESTRICTION CASE
CRN continued to defend evidence-based public health policy, consumer access to truthful information, and supplement marketers’ First Amendment rights, with its legal challenge to New York’s dietary supplement age-restriction law. Following a three-judge decision to deny CRN’s motion for a preliminary injunction in November, and the Second Circuit’s Dec. 30 denial of CRN’s petition for rehearing, CRN is preserving its options for further appeal.
At the heart of the case is the issue of whether the legislature can limit marketers’ and consumers’ First Amendment rights by imposing content-based restrictions on truthful claims about a supplement without having evidence that the incursion of free speech will accomplish the state’s purported health and safety interest.
In response to the court’s order, CRN filed a motion to stay the mandate of the Second Circuit in early 2026, seeking to pause further action in the case with time to seek further appeal to the U.S. Supreme Court. The motion argues that the Second Circuit’s decision lowers long-standing constitutional protections for truthful commercial speech by allowing speech-based restrictions without requiring the state to demonstrate, with evidence, that such limits materially advance public health or that less-restrictive alternatives were considered.
CRN is active in opposing state age restriction proposals in several states, most recently Michigan House Bill 5250, with Fox 2 Detroit's coverage including comments from CRN President & CEO Steve Mister.
CRN PROVIDES SCIENTIFIC CONTEXT AMID HIGH-PROFILE MEDIA COVERAGE
In October, CRN urged caution regarding a Consumer Reports study that tested protein powders for heavy metals, covered by several news outlets. As noted in CRN's statement, the mere detection of heavy metals such as lead, cadmium, or arsenic does not equate to a health hazard. CRN noted that supplement manufacturers are required under FDA Good Manufacturing Practices to test for contaminants and ensure compliance with federal standards. CRN comments were included in reporting from Today online, NPR and other syndicated coverage.
In addition, CRN responded to advance coverage of an American Heart Association Scientific Sessions presentation, calling for context when interpreting preliminary findings suggesting an association between long-term melatonin use and increased risk of heart failure. CRN advised that the early, non–peer-reviewed data cannot establish cause and effect and looked at study participants with chronic insomnia, which may itself be a contributing factor to heart health outcomes, raising more questions than answers. CRN highlighted its voluntary melatonin labeling guidelines as a resource, underscoring the upper dosage levels and advisory statements such as “For occasional and/or intermittent use only.”
CRN EDUCATES AND CONVENES EXPERTS ON EMERGING ISSUES
CRN’s members-only webinar on a new California law, effective next January, that imposes updated testing and disclosure requirements to prenatal (and postnatal) dietary supplements provided a roadmap of what to watch for with implementation of SB 646. CRN worked to mitigate the law’s impact on industry and is keeping members informed as California Department of Public Health issues guidance.
CRN’s industry-wide webinar on practical uses of AI in the dietary supplement industry brought together experts for a panel discussion on accelerating ingredient discovery and formulation, transforming regulatory and compliance operations and advancing scientific validation and consumer trust. Both webinars are now available on demand.
CRN ADVANCES SCIENCE-BASED SAFETY STANDARDS
 CRN published first-time safety reviews of key nutrients as part of the updates to its Vitamin & Mineral Safety book, now in its fourth edition. These include a new safety evaluation for choline and citicoline, essential nutrients for brain and liver health, and a new chapter on lutein, zeaxanthin, and meso-zeaxanthin, the carotenoids associated with vision and eye health.
CRN published first-time safety reviews of key nutrients as part of the updates to its Vitamin & Mineral Safety book, now in its fourth edition. These include a new safety evaluation for choline and citicoline, essential nutrients for brain and liver health, and a new chapter on lutein, zeaxanthin, and meso-zeaxanthin, the carotenoids associated with vision and eye health.
A new section on methylfolate, the biologically active form of folate, was added, while retaining the current tolerable Upper Intake Level (UL) for folate.
Q4 updates also included findings confirming the strong safety record of vitamin K2 (MK-7) supplements.
CRN PRESENTS FDA HUMAN FOODS HEAD FOR FIRESIDE CHAT AT BOARD MEETING
Kyle Diamantas, FDA Deputy Commissioner of Human Foods, participated in a Q&A session with CRN President & CEO Steve Mister during the association’s Q4 board of directors meeting. He participated in an “off the record” discussion of FDA’s priorities related to supplements and provided his perspectives on issues from the upcoming rulemaking on self-GRAS and the rationale behind FDA’s recent announcement on drug preclusion, to the influence the MAHA movement is having and the prospects for DSHEA reform.

CRN PROVIDES TARIFF INTEL, SUPPORT TO MEMBERS 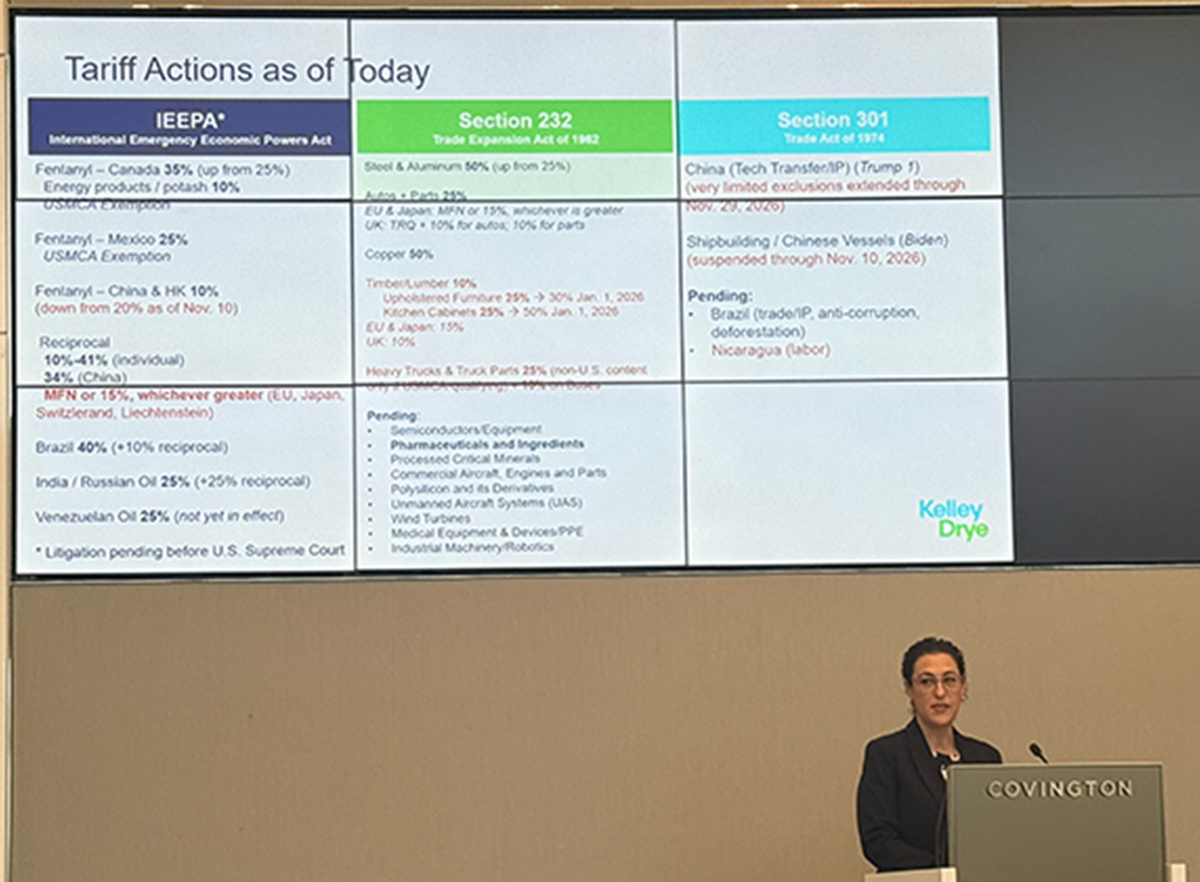
CRN has retained Brooke Ringel, with law firm Kelley Drye & Warren, to be available to CRN members as a no-cost member benefit to answer specific tariff and trade policy questions. At the association’s Q4 board meeting, Ringel discussed the tariff climate and navigating the ever-changing rates and rulings. She updated the Board on the recent Supreme Court arguments on the authority of the President to impose tariffs without Congressional approval, the other possible authorities available to the President if the Court rules against his current tariffs, and country-specific trade deals.
CRN CHARTS PATH FOR DIETARY SUPPLEMENT INDUSTRY AS FDA AND STATES PURSUE POLICY GOALS
In his address at CRN’s signature events and several end-of-year bylines President & CEO Steve Mister called for finding common ground with opponents and detractors, policy makers and regulators, MAHA allies and other stakeholders, while underscoring the importance of making industry’s voice heard in areas where it isn’t aligned with the government. He noted ways CRN has played defense in the states where CRN’s efforts have helped to:
- Exempt dietary supplements from legislation in Texas (Senate Bill 25) which would require warning labels on foods with certain additives
- Remove titanium dioxide from several state bills seeking to restrict certain ingredients
- Get clarification from California Governor Gavin Newsom that supplements wouldn’t be subject to testing and labeling requirements intended for baby food
- Secure amendments to a California bill requiring public disclosure of heavy metal test results for prenatal vitamins to allow for real-world context
- Successfully oppose age restrictions for weight management and sports nutrition products
CRN SUPPORTS FUTURE OF NUTRITION SCIENCE AND LEADERSHIP
CRN built connections between future nutrition leaders and responsible industry, again partnering with the American Society for Nutrition Foundation to award students with complimentary passes to the association’s Science in Session event. As shared in a recent episode of the Supplement Source podcast with scholars Huyen Le, Ben Levine, and past scholar Sam Fessler, who now works for CRN member company Needed, the program helps support the nutrition research community and broadens awareness of the role of supplements in nutrition and career opportunities in the supplement industry.

Huyen Le, left, is pursuing her Ph.D. in Nutritional Sciences, with a focus in nutritional biochemistry, at Rutgers University. Benjamin Levine, right, is pursuing his Ph.D. in Nutritional Sciences, with a focus in diet-host-microbiota interactions, at the University of Illinois.
In addition, through the Annette Dickinson Trailblazer Award, presented by CRN with Radicle Science, CRN also celebrated the leadership and pioneering work of professionals within its membership, this year honoring Deshanie Rai, Ph.D.
CRN President & CEO Steve Mister with Deshanie Rai, Ph.D., and Pelin Thorogood of Radicle Science.
GROWING OUR MEMBERSHIP
CRN welcomed new members including Voting members CJ Cheil Jedang Corporation (“CJ BIO”), Gnosis by Lesaffre, Perelel, and Univar Solutions, along with Associate members FoodChain ID, People Science and Pharmanager Development in Q4 2025.