
PRINTABLE PDF | SEE 2022 REPORTS
CRN kicked off its golden anniversary year in 2023 with a productive first quarter. We shared our policy priorities for the year in a webinar for CRN members and a bylined article in Natural Products Insider. Activation on these priorities is well underway, including developing solutions for state-level age restrictions, creating a legal pathway for CBD, expanding access to dietary supplements, engaging with our regulators at FDA—and much more. CRN hosted the CRN-I symposium in person again in conjunction with Codex in Düsseldorf, and presented educational webinars for our members on California's new packaging requirements and FTC's new guidance for products making health claims. Read on for highlights.
Celebrating #50yearsofCRN
CRN announced its 50thanniversary in 2023, noting each week we'd highlight a memory or milestone with a “Throwback Thursday” reflection on LinkedIn and Twitter, encouraging members to follow #50yearsofCRN on these platforms and save the date for CRN’s annual events, taking place Oct. 3–6 at the Ritz-Carlton, Laguna Niguel, in Dana Point, California. CRN also called for members to help make CRN history and join the "50 for 50" honor roll of 2023 sponsors.
Setting 2023 policy priorities
CRN staff reviewed the association's strategic plan adopted by the board of directors and developed policy priorities that effectuate the goals our members have set. The priorities for 2023 include the following and are explained in more detail here and in a member webinar with President & CEO Steve Mister (access presentation deck here) as well as a Natural Products Insider article:
- Expanding access to dietary supplements
- Making progress toward DSHEA modernization
- Creating a legal pathway for CBD
- Addressing drug preclusion
- Optimizing FDA reorganization
- Preparing for the final NDI Guidance
- Developing solutions for state-level age restrictions
- Engaging on sustainability solutions
Reaching international policymakers with CRN-I symposium
The CRN-International 2023 symposium convened academic experts from Canada, Germany, Italy, Norway, and the U.S. for presentations on advancing nutrition science to meet evolving global health needs, delivered to delegates in Düsseldorf for the Codex Committee on Nutrition and Foods for Special Dietary Uses (CCNFSDU) meeting and other international regulatory officials. As with previous CRN-I symposia, presenters will author a paper reviewing the science presented, slated for publication in the second quarter. Pictured here is Michelle Stout of Amway, who moderated the event, with presenters Dr. Marina Green, Dr. Lynette Neufeld, Dr. Charalampos Tzoulis, Dr. Emily Ho, Dr. Rima Obeid, and CRN's Dr. Jim Griffiths, who led the symposium program development.

Protecting the global probiotics market from a Codex proposal
CRN, in alignment with the International Alliance of Dietary/Food Supplement Associations (IADSA) has been steadfast in its opposition to a proposal before the Codex Committee on Nutrition and Foods for Special Dietary Uses (CCNFSDU) to create a global guideline for probiotics that could limit use of the word “probiotic.” During its March meeting in Düsseldorf, CCNFSDU agreed that conflicting perspectives on a probiotics harmonization proposal must be resolved prior to further advancement, citing issues regarding scope, definitions, external scientific advice, and potential work that would be required by other Codex Committees. The CCNFSDU directed that an electronic working group would refine and clarify the proposal with regards to the scope, impact on food safety and need for scientific advice to address the divergent expectations and views around resources needed to implement the proposal and whether this work is appropriate for the committee.
Developing solutions for state-level age restrictions—Maryland and more
During the first quarter, CRN’s government relations team engaged in opposition to several age-restriction proposals in state legislatures, keeping members updated on activity in California, Colorado, Massachusetts, Maryland, New Jersey, and New York. CRN President & CEO Steve Mister spoke with Natural Products Insider at Expo West in March about the legislation, also testifying in his home state of Maryland, along with the association's Craig Muckle, senior director of communications. In his testimony, Mister referenced a newly published scientific review commissioned by CRN, “Eating Disorders and Dietary Supplements: A Review of the Science,” which notes the lack of evidence to suggest a causative role for dietary supplements in eating disorders. CRN also provided a statement to the Associated Press, also cites the review, explaining, “Most experts agree there are multiple causes of eating disorders. However, there is an incontrovertible lack of evidence linking dietary supplements use to eating disorders, as demonstrated in the recent scientific review.” The Maryland legislation was tabled until next year; CRN continues to stay plugged into other state activity and monitoring the efforts of advocates for age-restriction legislation.
Promoting Congressional action on CBD
CRN applauded the introduction of the Hemp and Hemp-Derived CBD Consumer Protection and Market Stabilization Act of 2023 by Reps. Morgan Griffith (R-VA) and Angie Craig (D-MN) and said the association “remains committed to creating a legal pathway to market safe, non-psychoactive hemp-derived cannabinoids as dietary supplements.” A Hemp Roundtable statement included comment from CRN President & CEO Steve Mister saying, “Over four years ago, Congress purposefully removed hemp-derived CBD from the Controlled Substances List to jumpstart a market, yet FDA refuses to recognize that directive. While FDA delays, a sizable market has grown for CBD—legal or otherwise. Americans know these products are safe because they have been taking them for years. Consumers don’t need a new regulatory scheme that will potentially limit consumer choice.”
Earlier this year, CRN reacted to FDA’s denial of its 2020 citizen petition calling on the agency to create a regulatory pathway to legally market dietary supplements containing hemp-derived CBD and the agency’s separate announcement that FDA “has concluded a new regulatory pathway for CBD is needed that balances individuals’ desire for access to CBD products with the regulatory oversight needed to manage risks.”
CRN staff, with members on the CBD Working Group and Government Relations Committee, continue to express support for a legislative solution leading to recognizing CBD as a legal dietary ingredient.
Raising the profile of dietary supplements with new CRN comms lead, Jeff Ventura
CRN hired longtime healthcare communications strategist Jeff Ventura in February to lead the association's communications team. Ventura is developing a communications plan that will capitalize on the fact that 75% of Americans believe in the safety and efficacy of dietary supplements and will tell the macro story of supplements benefiting public health, while also including the stories of real individuals who are thriving using our industry’s products. The plan will also raise awareness and acceptance of supplements among health care providers, especially those working in community health settings and with populations that have difficulty accessing nutritious food and/or supplements. In addition to a proactive communications plan, Ventura is implementing new media relations tactics that have already resulted in CRN being included mainstream media coverage, such as Dr. Andrea Wong in USA Today on vitamin B. The team also kept CRN's Communications and Media Outreach Committee updated on major media coverage of dietary supplements and ingredients including ashwagandha, probiotics, and erythritol, along with the association's case-by-case response strategies.
Natural Products Expo West 2023 session explores supplements for brain health
CRN’s Natural Products Expo West 2023 educational session, Cognition Supplements for Optimal Performance, moderated by the association’s Luke Huber, N.D., featured presentations from Steven Kahn, Onnit; Diana Morgan, Nutrabolt; Caroline Davidson, SPINS; and Jeff Brams, Garden of Life.
CRN calls for FDA reform, provides feedback to agency officials
CRN submitted a letter to FDA Commissioner Robert Califf, M.D., in March following a January meeting with agency officials to discuss the Reagan-Udall Foundation report, “Operational Evaluation of the FDA Human Foods Program.” CRN called on the agency to prioritize dietary supplements and facilitate stronger collaboration and improved communication among its divisions and with industry stakeholders.
In addition, at FDA’s request, a meeting to discuss concerns regarding GMP facility inspections was held in February. In preparation for the meeting, CRN asked members to share examples of their inspection “headaches” to help inform the discussion with the agency about collaborating to develop solutions to inspection challenges and improve training of investigators. In the meeting, CRN emphasized the importance of addressing the broader issue of GMP compliance and asked to continue the dialogue on this issue with the goal of developing approaches that can be readily implemented.
FDA has announced a “Vision for a Reimagined FDA Human Foods Program,” which will foster more coordination between the policy divisions of the agency (the Center for Food Safety and Applied Nutrition and the Office of Dietary Supplement Programs) and the field inspectors (under the Office of Regulatory Affairs). If the reorganization occurs, it could further help centralize inspection priorities and allow more collaboration between the various divisions of FDA.
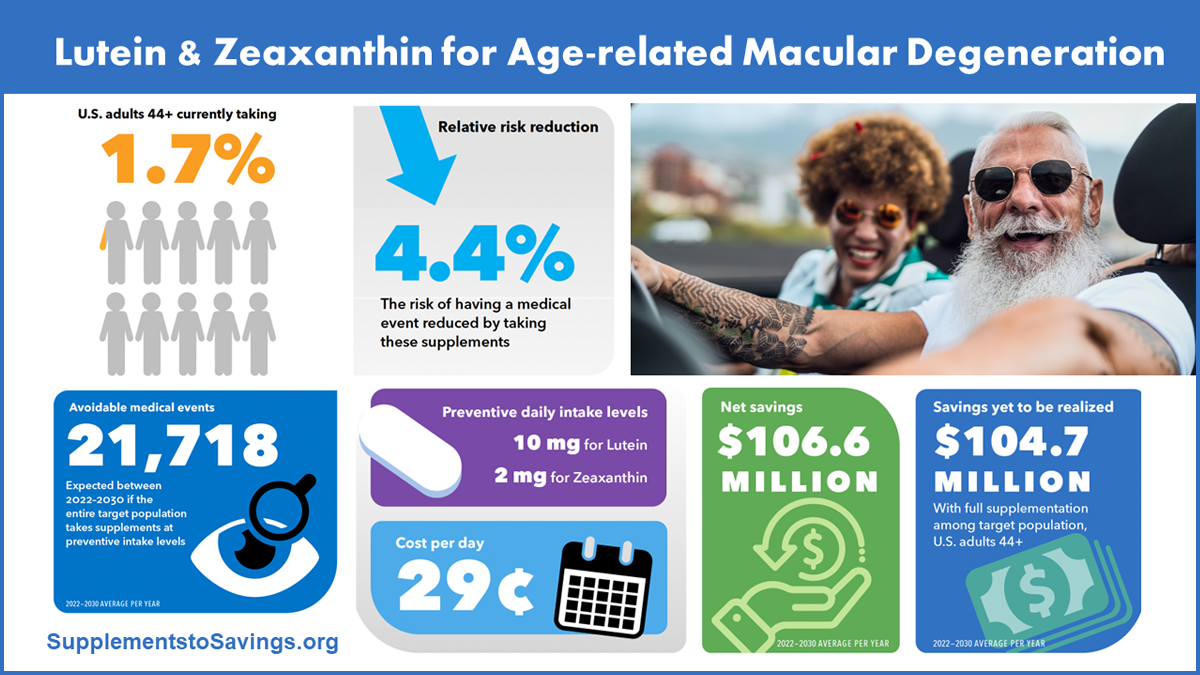
Highlighting 'Supplements to Savings' data on health care cost savings and quality of life improvement from dietary supplement use
CRN continued to share data from the CRN Foundation’s economic report, with first-quarter efforts featuring data from the chapters on optimal cognitive development, coronary artery disease, and age-related macular degeneration.
Mister attends White House hunger challenge kickoff event
CRN President & CEO Steve Mister in March attended the kickoff event for The White House Challenge to End Hunger and Build Healthy Communities, a nationwide call-to-action to make bold commitments to advance President Biden’s goal to end hunger and reduce diet-related diseases by 2030 while reducing disparities. The event included discussion with dignitaries such as Ambassador Susan Rice and chef José Andrés and follows up on federal and private sector initiatives announced during the historic September 2022 conference.
Expanding access to better nutrition and nutrition education to all Americans is one of CRN’s top policy priorities in 2023, as Mister explained in a bylined article for Natural Products Insider. “This year, CRN’s efforts to expand access will encompass a renewed push to include dietary supplements in Flexible Spending Accounts (FSAs) and Healthcare Savings Accounts (HSAs). We anticipate the introduction of legislation in Congress and substantive discussions to advance a bill through committee. In support of that effort, CRN’s newly released Supplements to Savings economic study demonstrates the monetary value of specific supplement regimens to lower healthcare costs. Additionally, we will invest in research to better understand how consumers leverage their FSAs and HSAs to lower healthcare expenses.”

Supplement OWL enhancements update model for listing
The Supplement OWL launched new “product carousel” feature appearing on a product’s page in the registry that allows a business user to display up to three images that site visitors may examine. Typical product carousel images for inclusion are facings of a bottle, product box, product in its packaging, and delivery format as well as delivery format size. The OWL, spearheaded by CRN in 2017, presents a model of dietary supplement listing, which the association frequently points out—most recently in its letter to FDA following up on the Reagan-Udall report. Participation in the Supplement OWL is mandatory for CRN members, who are encouraged to ensure their products are entered and regularly updated to include any new products and remove products that are discontinued.
Keeping members updated on class action risk and regulatory considerations
CRN is monitoring a concerning litigation trend regarding overages, with an increase in class actions targeting this practice. Prior to 2022, we were aware of only a few class actions related to overages. In 2022, however, at least five cases were filed alleging that overage amounts for melatonin in dietary supplement products were unreasonable and deceptive to consumers. This is a determination, however, that should be left to FDA—not left for individual courts to interpret under state law—under the legal theory that FDA’s regulations preempt state laws. CRN raised concerns with FDA about courts’ unwillingness to give deference to FDA jurisdiction in these and other situations, such as DSHEA disclaimer placement and BCAA calorie labeling. CRN will also continue to discuss class action trends in-depth with its Legal Committee to alert members of emerging risks.
Presenting educational content for CRN members
In addition to a webinar presentation of CRN's 2023 policy priorities, the association hosted two educational webinars for its members in the first quarter on:
- FTC Health Products Compliance Guidance Updates
- California’s Plastic Pollution Prevention and Packaging Producer Responsibility Act
Commenting on regulatory issues affecting dietary supplements
CRN collaborated with members on feedback to FDA and the U.S. Preventive Services Task Force (USPSTF) on the following items:
CRN noted its support of FDA’s proposal to formally exempt clinical studies conducted on food and dietary supplements from IND application requirements if they are not intended to support marketing of the products as a drug, but called for clarification on several areas of the proposal, calling on the agency to withdraw all parts of its final guidance on INDs related to food and dietary supplements until rulemaking is completed.
FDA's Proposed Rule on Food Labeling: Nutrient Content Claims; Definition of Term “Healthy”
CRN emphasized the value of including dietary supplements and functional foods in the final rule and urged the agency to reconsider its “food groups-only” approach, noting an additional approach based on "nutrients to encourage" and "nutrients to limit" is needed to allow the “healthy” claim on a range of formulated foods and dietary supplements can contribute to overall consumption of a variety of nutrients important for maintaining and supporting good health.
CRN recommended that the proposed analytic framework be modified to reflect vitamin D status as a central component of the research plan, noting vitamin D status as a critical component of any research that is conducted to investigate the relationship between vitamin D and health outcomes.
Reminder—save the date for CRN's annual events
Mark your calendar and plan on joining us Oct. 3–6 at the Ritz-Carlton, Laguna Niguel for our annual events, Science in Session and Now, New, Next—including a 50th anniversary celebration!



