A topline report from the Council for Responsible Nutrition (CRN),
the leading trade association for the dietary supplement and functional food industry
Congress affirms #SarmsCanHarm
 Senators Orrin Hatch (R-UT) and Sheldon Whitehouse (D-RI) last month introduced S 2742, the SARMs Control Act of 2018, in the U.S. Senate, taking action to protect consumers from Selective Androgen Receptor Modulators (SARMs) that can show up in product mislabeled as dietary supplements. The bill provides the Drug Enforcement Administration (DEA) the authority to remove illegal products containing SARMs from the market as controlled substances.
Senators Orrin Hatch (R-UT) and Sheldon Whitehouse (D-RI) last month introduced S 2742, the SARMs Control Act of 2018, in the U.S. Senate, taking action to protect consumers from Selective Androgen Receptor Modulators (SARMs) that can show up in product mislabeled as dietary supplements. The bill provides the Drug Enforcement Administration (DEA) the authority to remove illegal products containing SARMs from the market as controlled substances.
Consumers are put at risk and the dietary supplement industry’s reputation is damaged by companies illegally marketing these unapproved drugs as dietary supplements. In addition to advocating for legislation like S 2742, CRN is leading an education initiative to raise awareness among consumers, specifically those in the bodybuilding and fitness communities, about the dangers of SARMs. Retailers are invited to join the movement by clicking here.
Supplements may not claim to protect consumers from sun exposure
 FDA announced agency-wide action to protect consumers from the harmful effects of sun exposure, including several warning letters for firms marketing dietary supplements with disease claims. FDA warns that these products, including Advanced Skin Brightening Formula, Sunsafe Rx, Solaricare and Sunergetic, put people at risk by giving consumers a false sense of security that a dietary supplement could prevent sunburn or protect from the risks of skin cancer. FDA Commissioner Scott Gottlieb, M.D., released a statement to summarize broader agency actions.
FDA announced agency-wide action to protect consumers from the harmful effects of sun exposure, including several warning letters for firms marketing dietary supplements with disease claims. FDA warns that these products, including Advanced Skin Brightening Formula, Sunsafe Rx, Solaricare and Sunergetic, put people at risk by giving consumers a false sense of security that a dietary supplement could prevent sunburn or protect from the risks of skin cancer. FDA Commissioner Scott Gottlieb, M.D., released a statement to summarize broader agency actions.
Representing responsible industry, CRN joined the conversation on Twitter and LinkedIn, and was even retweeted by Dr. Gottlieb himself.
FDA takes action against harmful products containing pure caffeine
 “Pure caffeine has no place in the consumer marketplace,” said CRN in response to a new guidance from FDA, “Highly Concentrated Caffeine in Dietary Supplements; Guidance for Industry; Availability.” The guidance, applauded by CRN, puts all dietary supplement stakeholders, including online retailers, on notice that highly-concentrated caffeine sold in bulk to consumers is dangerous and illegal. According to CRN, the guidance illustrates FDA’s commitment to taking immediate steps to remove products from the marketplace that present public health concerns. A statement by CRN’s Steve Mister regarding FDA’s guidance appeared on NBC Nightly News, in USA Today, and in several other media outlets.
“Pure caffeine has no place in the consumer marketplace,” said CRN in response to a new guidance from FDA, “Highly Concentrated Caffeine in Dietary Supplements; Guidance for Industry; Availability.” The guidance, applauded by CRN, puts all dietary supplement stakeholders, including online retailers, on notice that highly-concentrated caffeine sold in bulk to consumers is dangerous and illegal. According to CRN, the guidance illustrates FDA’s commitment to taking immediate steps to remove products from the marketplace that present public health concerns. A statement by CRN’s Steve Mister regarding FDA’s guidance appeared on NBC Nightly News, in USA Today, and in several other media outlets.
Retailers rejoice: restrictive bill pulled from Massachusetts House floor
 CRN bids farewell to Massachusetts HB 1195, a bill that would have banned the sale of weight-loss and muscle-building dietary supplements to minors and placed an economic burden on Massachusetts’ retailers. Pulled from the House floor by the Massachusetts Legislature, the bill was sent to a study by the Joint Committee on Public Health (JCPH), effectively ending any prospect for passage during this legislative session.
CRN bids farewell to Massachusetts HB 1195, a bill that would have banned the sale of weight-loss and muscle-building dietary supplements to minors and placed an economic burden on Massachusetts’ retailers. Pulled from the House floor by the Massachusetts Legislature, the bill was sent to a study by the Joint Committee on Public Health (JCPH), effectively ending any prospect for passage during this legislative session.
CRN aggressively fought against this legislation since it was first introduced in 2015. Partnering with like-minded organizations (including Massachusetts retailers), CRN worked with a strong coalition and testified twice before the JCPH to defend consumer access to safe dietary supplements and to highlight the ways in which the bill does not achieve any clear public health objective.
For more information, contact Ingrid Lebert (202-204-7699).
CRN to FDA: ‘increased intelligence is only valuable if used appropriately’
An announcement from FDA Commissioner Scott Gottlieb, M.D., noted the agency’s adoption of new advanced screening technologies at ports of entry to allow inspectors to screen packages containing suspected drug products more efficiently and reliably. CRN applauded the move, noting in a statement that “responsible industry has been adamant about the need for FDA to increase the effectiveness of its regulation of dietary supplements […] and is committed to support the agency’s efforts to increase consumer safety through the prevention of illegal products from entering the marketplace.”
However, CRN warned against “taking FDA’s findings out of context and making generalizations about the dietary supplement industry as a whole.”
 Short and Tweet
Short and Tweet
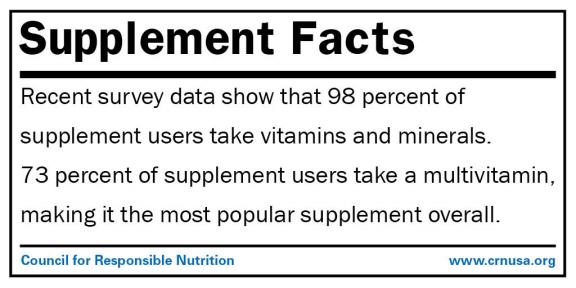
Know your #SupplementFacts