Past Editions—Need to Know Archives
‘Supplements to Savings’ report demonstrates billions in savings from the use of dietary supplements
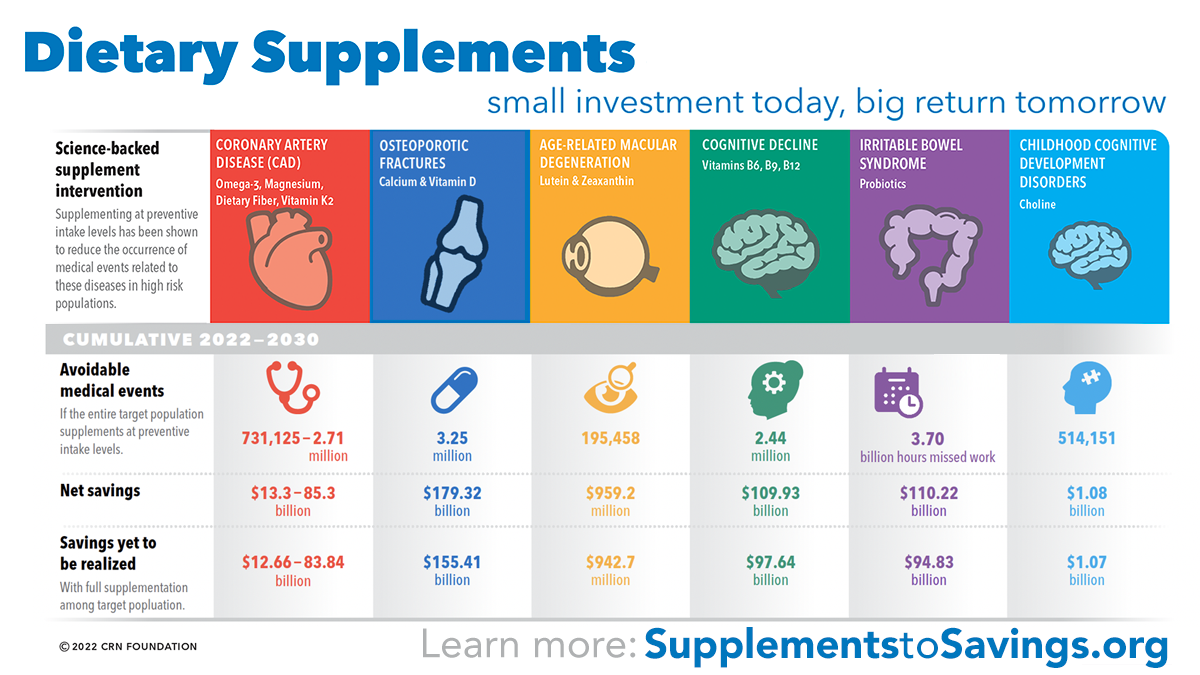
A new economic report, “Supplements to Savings: U.S. Health Care Cost Savings from the Targeted Use of Dietary Supplements, 2022–2030,” details how billions of dollars in U.S. healthcare costs can be realized through the use of specific supplements by at-risk populations.
“Supplements to Savings” presents data showing a reduction of direct and indirect medical costs associated with several chronic diseases and conditions—coronary artery disease, osteoporosis, age-related macular degeneration, cognitive decline, irritable bowel syndrome, and childhood cognitive development disorders—from the use of specific supplement regimens.
“Identifying at-risk populations early and providing targeted nutritional interventions like dietary supplements is a cost-effective approach,” to lowering societal health care costs, said CRN Senior Vice President of Scientific and Regulatory Affairs Andrea Wong, Ph.D.
The Supplements to Savings report:
-
Estimates $59 billion in health care cost savings at current supplementation rates.
-
Reveals the nine supplements and intake levels needed to realize the additional billions in healthcare savings.
-
Explains the target populations for supplementation.
By the numbers: More than 75% of the nation’s healthcare spending is for people with chronic conditions, according to the U.S. Centers for Disease Control and Prevention. In addition to healthcare spending, these chronic diseases cost the U.S. more than $260 billion annually in lost workforce productivity. The U.S. invests less than 3% of total healthcare expenditures on preventive care services.
Stakeholders—including retailers—are encouraged to share data from the report, with individual chapters and accompanying infographics available to highlight key findings by ingredient, including omega-3 fatty acids, magnesium, soluble fiber, vitamin K2, calcium and vitamin D, lutein and zeaxanthin, vitamins B6, B9, and B12, probiotics, and choline. Tell your consumers the good news about dietary supplements for their wallets!
New data released from the CRN Consumer Survey on Dietary Supplements

Supplement usage is back to pre-pandemic levels, according to new data from the 2022 CRN Consumer Survey on Dietary Supplements, released Oct. 13—and which is also available for purchase.
By the numbers: Overall, 75% of Americans report using nutritional or dietary supplements.
-
70% report taking a multivitamin in the past 12 months.
-
77% of Americans find the industry trustworthy—that percentage is even higher among supplement users, at 84%.
-
52% of supplement users report taking a specialty supplement—the most reported of which are omega-3s, probiotics, melatonin, and fiber.
“This year’s data shows a remarkable picture of continuity and paints the landscape of a vibrant, mainstream industry,” said Brian Wommack, CRN’s senior vice president of communications in NutraIngredients-USA. “While overall usage is down slightly from its pandemic peak, reported usage of immunity-boosting supplements—including vitamin D, vitamin C, and zinc—is steady from last year.”
For the second year in a row, the CRN survey includes oversamples of Black, Hispanic, and Asian/Pacific Islander respondents to ensure significant sample sizes of supplement users of color and provide deep insights into the groups’ motivations, attitudes, and purchasing habits.
Buy the numbers: The full dataset from the 2022 CRN Consumer Survey is now available for purchase for $7,500.
Gov. Newsom vetoes supplement age-restriction legislation

California Governor Gavin Newsom last month vetoed AB 1341, legislation that would have placed age restrictions on retail sales of certain weight-loss supplements.
CRN reacted to the news, noting its Government Relations team’s work spanning more than a year to secure changes to the final proposal that would narrow the bill’s scope and protect its members’ legitimate products. Ultimately, those changes prompted Newsom’s veto instead.
“CRN had multiple conversations and a good working relationship with bill author Assemblymember Cristina Garcia and her staff,” said CRN VP Government Relations Julia Gustafson. “We appreciate her willingness to include practical measures in the final version of her legislation that limited its scope and removed behind-the-counter restrictions.”
In his veto message, Gov. Newsom lamented that the bill would “require [the Dept of Public Health] to evaluate every individual weight loss and dietary supplement product for safety.” In vetoing the bill, he wrote that his administration will “work with the legislature next year to create legislation that does not require the state to undertake lengthy and costly pharmacological studies on the many supplements on the market today.”
What’s next: “We expect this bill or similar proposals to be reconsidered during the next legislative session,” said Gustafson. “CRN stands ready to work with the lawmakers to ensure this legislation is reintroduced in a way that responsibly balances consumer safety with public access to dietary supplements.”
Mandatory product listing update from Washington

“CRN remains committed to the creation of a mandatory registry for dietary supplements, and the greater insight it will give to FDA as regulator and the transparency it will offer to all consumers,” said CRN VP, Government Relations, Julia Gustafson, in a statement issued in response to the release of the text of the Continuing Appropriations and Ukraine Supplemental Appropriations Act of 2023—and the indication that legislation will advance without including provisions to increase transparency for dietary supplements.
As reported by NutraIngredients, Gustafson noted CRN is “disappointed that, due to time constraints and the need to produce a ‘clean bill’ that can win congressional approval at this eleventh hour, leaders of both parties decided remove all of the ‘super riders’ that were included by the U.S. Senate to this ‘must-pass’ legislation.”
What’s next: “Already, conversations are occurring on Capitol Hill about other possible vehicles to enact MPL before the end of this Congress, and we look forward to continuing our seat at these negotiations,” explained Gustafson.
The bottom line: “Today’s events are a setback, but not the end to our effort to provide FDA with visibility into a marketplace it is charged with regulating," said Gustafson.
“We are a $56 billion industry and that calls for being responsible in ways that weren’t required 28 years ago,” CRN President & CEO explained during his state of the industry address at the association’s annual conference.
“We can’t rely on the old ways of defending ourselves in this new environment. The solution goes beyond just demonstrating how we are already regulated to considering what new tools we can give our regulators that assure them our products don’t create unreasonable risk. And of course, mandatory product listing is at the top of that list—it just makes common sense to give the agency the ability to know what’s being sold in the marketplace.”
Stay updated on MPL: www.crnusa.org/MPL